Advancing CAR-T Therapy: Regen BioPharma’s Promising Pre-Clinical Validation Journey
SAN DIEGO, Sept. 13, 2022 /PRNewswire/ — Regen BioPharma, Inc. (OTC PINK: RGBP) and (OTC PINK: RGBPP) announced today initiation of a series of phased experiments to begin the process of moving its CAR-T cell de-differentiation approach through pre-clinical validation. CAR-T cells are T cells (the lymphoid cells of the body that kill tumors) isolated from a cancer patient that have been modified by expressing a chimeric antigen receptor (CAR) which is specific for the patient’s tumor.
 While CAR-T cells are effective at treating certain lymphomas and leukemias, solid tumors such as liver, breast and colon remain resistant to CAR-T therapies for several reasons. One reason is “T cell exhaustion”, a term that means the T cells that are initially recruited to the tumor to kill it end up losing their effectiveness.
While CAR-T cells are effective at treating certain lymphomas and leukemias, solid tumors such as liver, breast and colon remain resistant to CAR-T therapies for several reasons. One reason is “T cell exhaustion”, a term that means the T cells that are initially recruited to the tumor to kill it end up losing their effectiveness.
The company believes that NR2F6, a checkpoint that puts the brakes on T cell activity, is a key player in the T Cell exhaustion phenomenon. Inhibiting NR2F6 is expected to prevent these T cells from becoming dysfunctional.
The Company has engaged the contract research organization, ProMab Biotechnologies, Inc. of Richmond, California, to embark on a series of experiments using the Company’s proprietary shRNA NR2F6-inhibiting technology to validate this approach.
“We are extremely excited to be using our cutting-edge genetic approach to create long-lasting CAR-T cells,” says David Koos, Chairman and CEO of the Company. “By partnering with a well-qualified organization such as ProMab Biotechnologies, Inc., we expect to quickly move this program forward to the clinic.”
Regen BioPharma, Inc. Begins Experiments Validating Its Proprietary CAR-T Cell Therapy (prnewswire.com)
Charting Intellectual Property Ventures: RGBP’s Expansive Licensing Initiatives

On July 26th RGBP announced the filing with the United States Patent and Trademark Office of a provisional patent application covering utilization of dendritic cell technologies to augment efficacy of its patented survivin mRNA cancer immunotherapeutic vaccine.
RGBP owns a valuable intellectual property portfolio including eight issued patents and 13 published patents. Zander Therapeutics Incorporated has been granted an exclusive license to develop and commercialize IP controlled by the company for non-human veterinarian therapeutic use applications.
Regen has granted an exclusive license to Oncology Pharma, Inc. to develop and commercialize “Antigen specific mRNA cellular cancer vaccines” for the treatment of pancreatic cancer and KCL Therapeutics, Inc. has granted an exclusive license to Oncology Pharma, Inc. to develop and commercialize certain intellectual property for the treatment of colon cancer. Regen BioPharma, Inc. Responds to Numerous Requests for Updates on its Intellectual Property (IP) Portfolio (prnewswire.com)
Technical breakdown
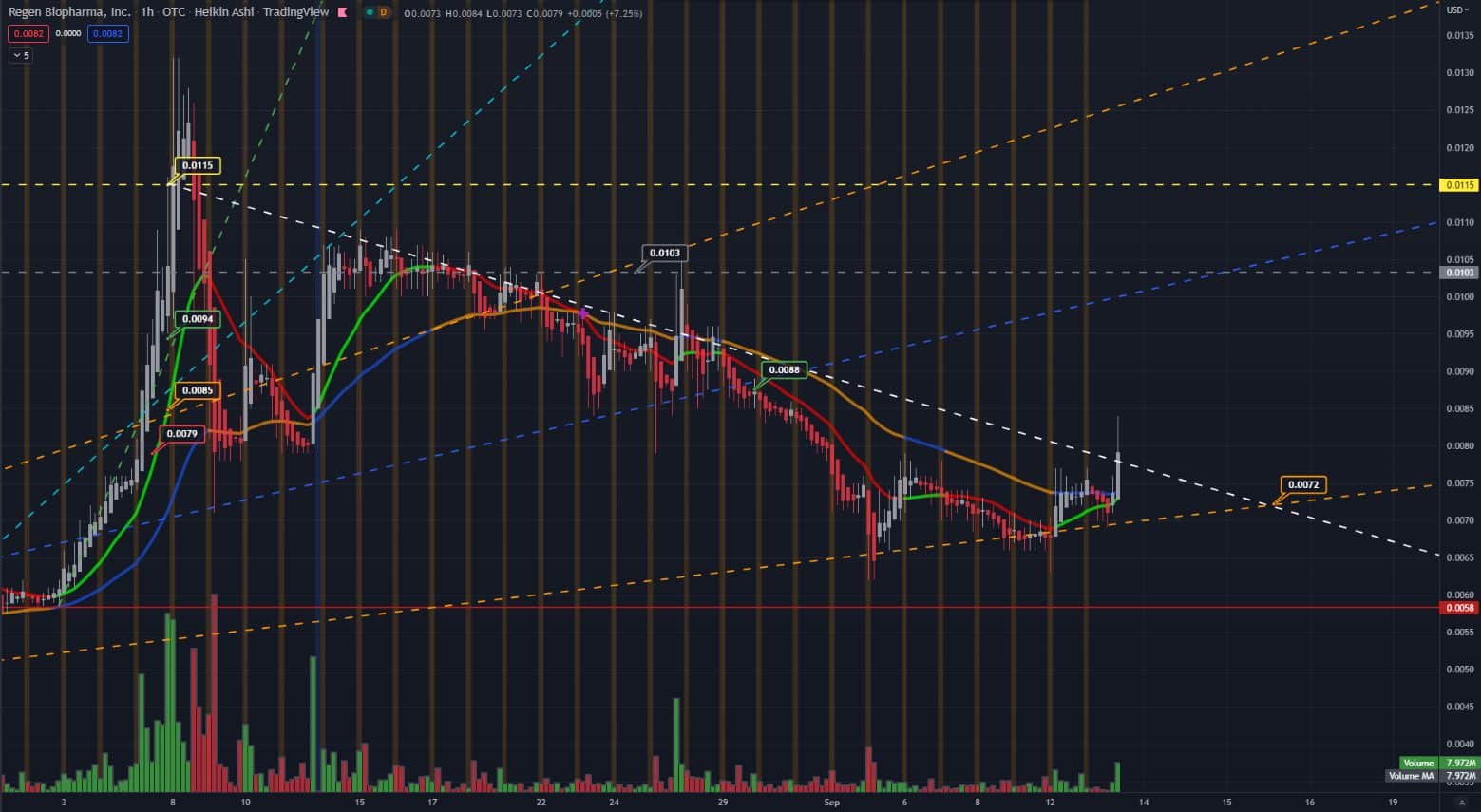
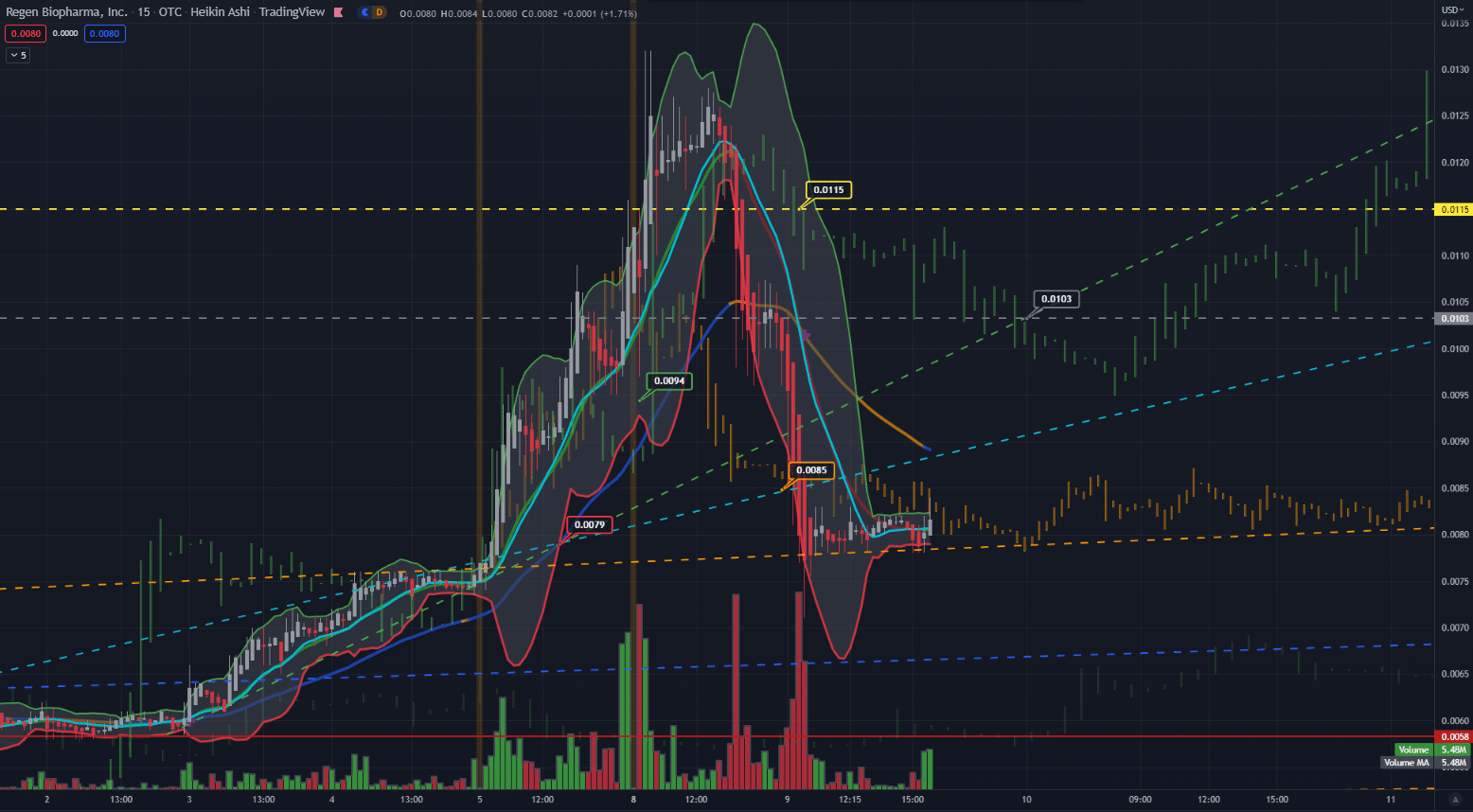 Chart and analysis from our article August 9th RGBP – Regen Biopharma, Inc ‣ TradersQue ‣ September 2022
Chart and analysis from our article August 9th RGBP – Regen Biopharma, Inc ‣ TradersQue ‣ September 2022
“Since July 20th. The stock moved from $.004 to $.0075. Friday August 5th RGBP had an excellent move breaking into the $.01 territory. Monday August 8th the stock continued to climb from just under a penny at $.0098 to a total resistance high of $.0132. Tuesday the stock retraced 17.53% to a low of $.0071 and held a solid weekly trend around the $.0072 area.” RGBP – Regen Biopharma, Inc. | Security Details | OTC Markets
Tuesday September 13th confirms a trend pivot from a retrace to a bullish pattern. Hourly resistance sits at .0088, Daily resistance is .0103 and a lower support of .0072 remains. Today’s news had a positive impact on shareholders and shows signs of continued transparency and updates from RGBP can be expected. “ProMab Biotechnologies, Inc. of Richmond, California, will embark on a series of experiments using the Company’s proprietary shRNA NR2F6-inhibiting technology to validate this approach.”