VistaGen is revolutionizing mental health with groundbreaking treatments for anxiety, depression, and CNS disorders. Their cutting-edge approach and pioneering Pherine technology promise faster, safer therapies.
VistaGen (Nasdaq: VTGN) is an advanced biopharmaceutical company focused on revolutionizing anxiety, depression, and CNS disorder treatments.Specializing in neuroscience, VistaGen develops faster-acting, safer treatments with therapeutic promise across various CNS modalities and markets.
Revolutionizing Mental Health
To achieve this, VistaGen maintains a clinical pipeline with differentiated mechanisms of action (MOAs) and modalities targeting significant markets in anxiety, depression, and other neuroscience domains.
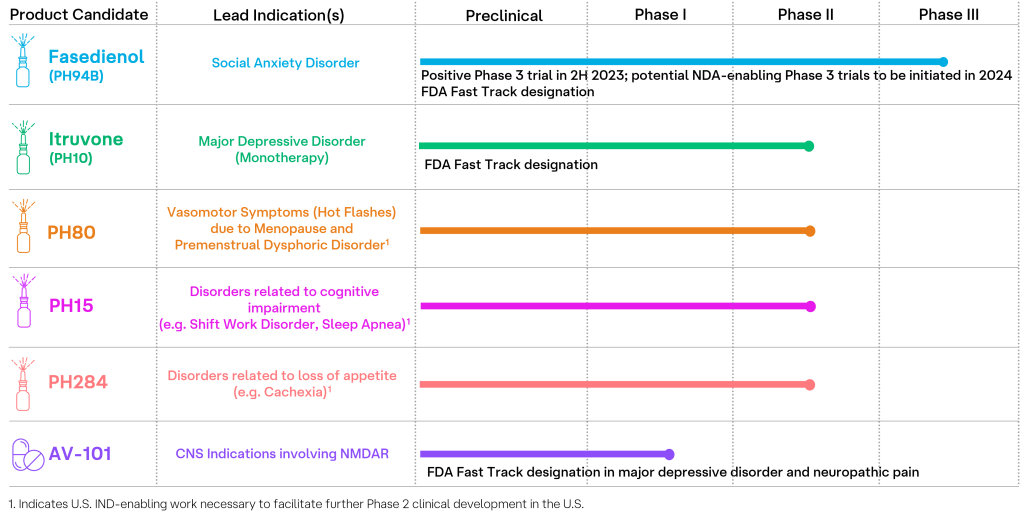
The pipeline comprises six clinical-stage products: Fasedienol (PH94B), Itruvone (PH10), PH80, PH15, PH284, and AV-101, with the latter five utilizing Pherine technology. Pherines are neuroactive nasal sprays that activate chemosensory neurons, exhibiting rapid activation, local metabolization (i.e., requiring no brain or blood uptake), and lacking both disagreeable odo and tastes while maintaining favorable safety profiles compared to traditional prescriptions, including common SSRIs.
VistaGen as a Visionary
VistaGen’s core corporate principles—integrity, compassion, teamwork, and excellence—underlie a pipeline addressing various CNS disorders, such as vasomotor symptoms, cognitive impairment, appetite loss, and neuropathic pain. AV-101, recently patented by the European Patent Office for neuropathic pain treatment, positions VistaGen advantageously until 2034, following FDA fast-tracked domestic clinical trials. AV-101, an investigational oral pro-drug developed by the company, has shown robust effects similar to gabapentin but with a superior side effect profile, as demonstrated in preclinical models of pain associated with inflammation and nerve injury.
VistaGen’s leadership team, including those with personal experiences of CNS disorders, actively contributes to compassionate community care. Recognizing the need for collaboration, VistaGen fosters a team culture valuing collective achievement through excellence in healthcare innovation. VistaGen also boasts clinical and regulatory advisors from prestigious institutions like Yale, Harvard, the U.S. FDA, Columbia, UC San Diego, and Baylor.
Pipeline Product Applications
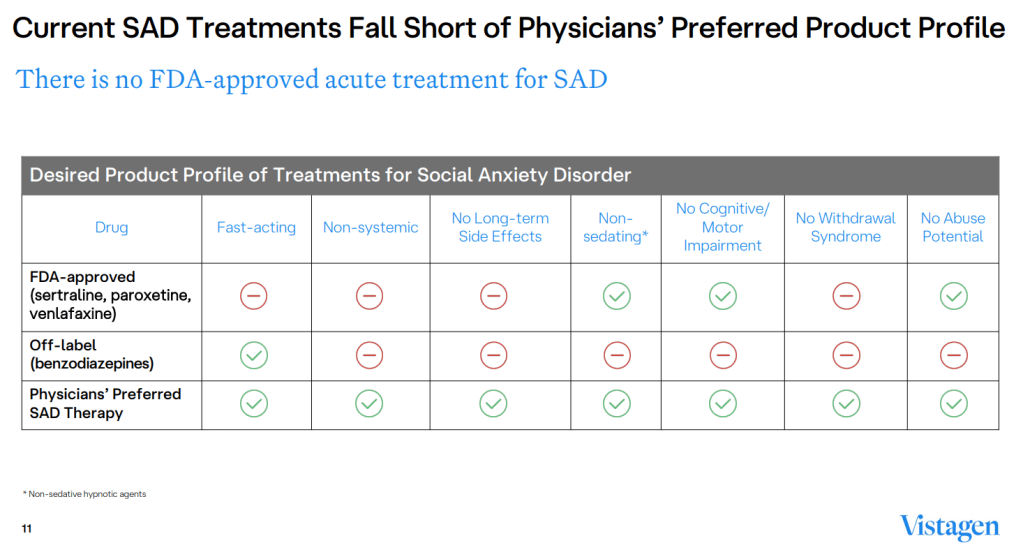
Social Anxiety Disorder (SAD) affects approximately 15 million U.S. adults, with symptoms emerging around age 13. Public speaking and meeting new people prove difficult, if not near impossible, for those living with SAS. Diminishing its effects is VistaGen’s Fasedienol, a rapid-onset, investigational Pherine-based nasal spray designed to treat multiple anxiety disorders without the side effects of current medications.
Depression, impacting 21 million U.S. adults, is mitigated by VistaGen’s Itruvone, a rapid-onset, investigational Pherine-based nasal spray serving as a potential stand-alone treatment for major depressive disorder and other neuro-psychiatric condition associated with depression. With 10 percent of the U.S. adult population reporting at least one major depressive crisis or episode annually, Itruvone can prove revolutionary to scale.
Anxiety, depression, and other CNS disorders significantly impact daily life, leading to chronic disruption, chronic health effects, and a higher mortality rate if left untreated. Quality of life decreases by up to 25 years without sufficient treatment.

Additional Pipeline Product Applications
For Menopausal Hot Flashes, Premenstrual Dysphoric Disorder and Migraine, PH80, an investigational Pherine, is a potential treatment for the acute management of vasomotor symptoms (hot flashes) due to menopause, acute management of symptoms of premenstrual dysphoric disorder and acute treatment of migraine.
For Improvement of Cognition, PH15 is an investigational Pherine designed as a potential new treatment for many disorders that lead to sleep deprivation and ensuing fatigue and cognitive impairment.
For Appetite-related Disorders, PH284 is an investigational Pherine with potential to treat appetite related disorders such as Cachexia (Wasting Syndrome), a serious but underrecognized consequence of many chronic diseases and advanced cancer.
Corporate Update
 VistaGen’s CEO, Shawn Singh, expressed confidence in the company’s achievements, highlighting significant milestones, including positive Phase 3 results for Fasedienol, their lead Pherine nasal spray drug candidate. The successful PALISADE-2 Phase 3 study demonstrated Fasedienol’s efficacy in treating anxiety in adults with social anxiety disorder (SAD), meeting primary, secondary, and exploratory endpoints.
VistaGen’s CEO, Shawn Singh, expressed confidence in the company’s achievements, highlighting significant milestones, including positive Phase 3 results for Fasedienol, their lead Pherine nasal spray drug candidate. The successful PALISADE-2 Phase 3 study demonstrated Fasedienol’s efficacy in treating anxiety in adults with social anxiety disorder (SAD), meeting primary, secondary, and exploratory endpoints.
Second Quarter Financial Results for Q2 FY2024
Research and Development (R&D) expenses decreased by approximately $9.0 million, going from $12.9 million in the quarter ended September 30, 2022, to $3.9 million in the same period of 2023. This reduction is mainly attributed to the completion of initial studies in the PALISADE Phase 3 Program for Fasedienol in social anxiety disorder (SAD), along with decreased nonclinical development, regulatory and outsourced manufacturing, and regulatory activities for Fasedienol and Itruvone.
General and Administrative (G&A) expenses decreased by $500,000, dropping from $3.7 million for the quarter ended September 20, 2022, to $3.2 million for the quarter ended September 30, 2023. This decline is primarily due to decreased compensation, consulting, and professional services.
Net Loss attributable to common stockholders for the second quarter ended September 30, 2023, was approximately $6.6 million, a significant decrease from the net loss of $17.5 million recorded on September 30, 2022.
Cash Position: As of September 30, 2023, the company held approximately $37.6 million in cash and cash equivalents. Additionally, since that date, VistaGen received about $93.5 million in net proceeds from an underwritten public offering of its equity securities and $1.5 million from Fuji Pharma for an exclusive negotiation agreement regarding a potential license to develop and commercialize PH80 in Japan. With the success of the PALISADE Phase 3 Program, the company believes its current cash position will be adequate to fund operations through the potential submission of a U.S. New Drug Application for Fasedienol in the first half of 2026.
As of November 9, 2023, the company had 27,023,038 shares of common stock outstanding.

Anticipated Positive Developments
In response to the positive outcomes observed in PALISADE-2, VistaGen is gearing up to launch two additional Phase 3 clinical trials of Fasedienol in 2024. These trials, known as PALISADE-3 and PALISADE-4, are designed with an Open-label Extension (OLE) approach.
The Phase 3 Acute Treatment Public Speaking Challenge in PALISADE-3 mirrors the setup of PALISADE-2, while the potential OLE aspect could extend for up to 12 months. The projected timeline involves initiating PALISADE-3 in the first half of 2024 and PALISADE-4 in the second half of the same year. Each Phase 3 study aims for an enrollment of approximately 230 participants, although the initiation of these trials is contingent on obtaining feedback from the FDA.
VistaGen anticipates that the success of either PALISADE-3 or PALISADE-4, when combined with the positive results from PALISADE-2, could establish substantial evidence supporting the effectiveness of Fasedienol. This collective evidence may then serve as the basis for a potential submission of a U.S. New Drug Application (NDA) to the FDA for the acute treatment of anxiety in adults with Social Anxiety Disorder (SAD) in the first half of 2026.
Staying Updated
For more comprehensive insights into VistaGen’s journey, visit Daily Q ‣ TradersQue ‣ Elevate your trading game today! for ongoing coverage. Additional topics and perspectives are available on the author’s LinkedIn page.
For more detailed information, refer to VistaGen’s official website at www.vistagen.com.


